Reduction of Lead Oxide (PbO2) by Iodide and Formation of Iodoform in the PbO2/I−/NOM System | Environmental Science & Technology
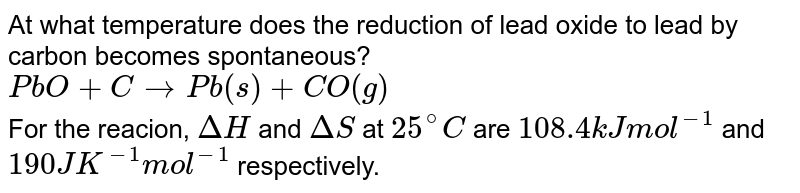
At what temperature does the reduction of lead oxide to lead by carbon becomes spontaneous? PbO +C rarr Pb(s) +CO(g) For the reacion, DeltaH and DeltaS at 25^(@)C are 108.4 kJ mol^(-1)

The Release of Lead from the Reduction of Lead Oxide (PbO2) by Natural Organic Matter | Environmental Science & Technology

For the reduction of lead oxide by coke `(PbO+C rarr Pb+CO), Delta H` and `Delta S` are found to be - YouTube

The Release of Lead from the Reduction of Lead Oxide (PbO2) by Natural Organic Matter | Environmental Science & Technology

The metal whose oxide, which is amphoteric, is reduced to metal by carbon reduction . (Fe/ Mg/ Pb/ Al) .
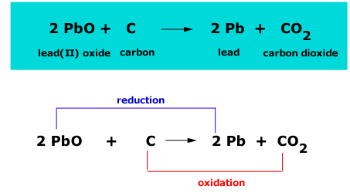
![For the given reaction,\\[2PbO + C \\to Pb + C{O_2}\\] name the oxidized substance, reduced substance, reducing agent, and oxidizing agent in this reaction. For the given reaction,\\[2PbO + C \\to Pb + C{O_2}\\] name the oxidized substance, reduced substance, reducing agent, and oxidizing agent in this reaction.](https://www.vedantu.com/question-sets/407a8186-d96f-4ae5-9b1b-a35a89f410b32918307568284777948.png)
For the given reaction,\\[2PbO + C \\to Pb + C{O_2}\\] name the oxidized substance, reduced substance, reducing agent, and oxidizing agent in this reaction.