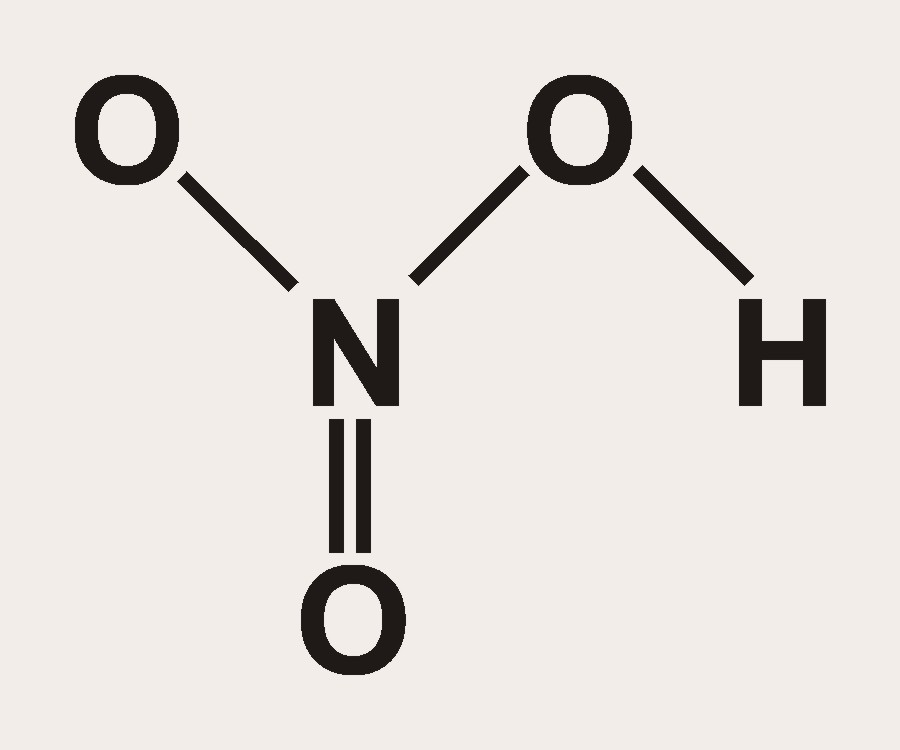
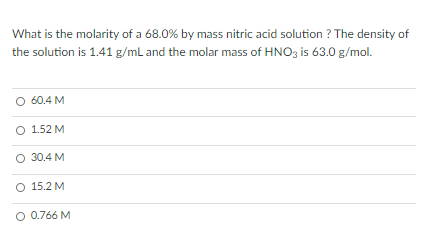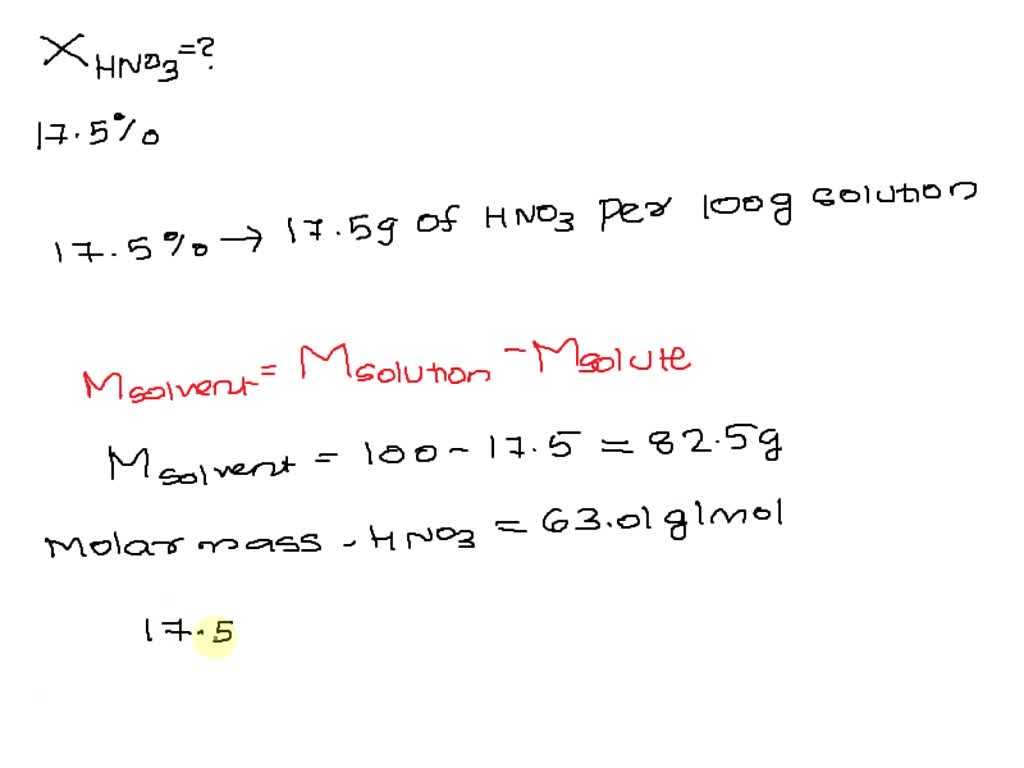How many grams of concentrated nitric acid solution should be used to prepare 250 mL of 2.0 M HNO3 ? The concentrated acid is 70
![SOLVED: The concentration of a nitric acid (HNO3) solution is 54.2% by mass. Calculate the molality (in mol/kg) of HNO3. (Hint: Start with an assumption.) [MM of HNO3 is 63.02 g/mol] SOLVED: The concentration of a nitric acid (HNO3) solution is 54.2% by mass. Calculate the molality (in mol/kg) of HNO3. (Hint: Start with an assumption.) [MM of HNO3 is 63.02 g/mol]](https://cdn.numerade.com/ask_previews/28732749-7e1f-49c5-ba82-98b8229d29aa_large.jpg)
SOLVED: The concentration of a nitric acid (HNO3) solution is 54.2% by mass. Calculate the molality (in mol/kg) of HNO3. (Hint: Start with an assumption.) [MM of HNO3 is 63.02 g/mol]

24. Calculate the molar mass of (a) water (H20) (b) nitric acid (HNO3).with full steps. - Brainly.in

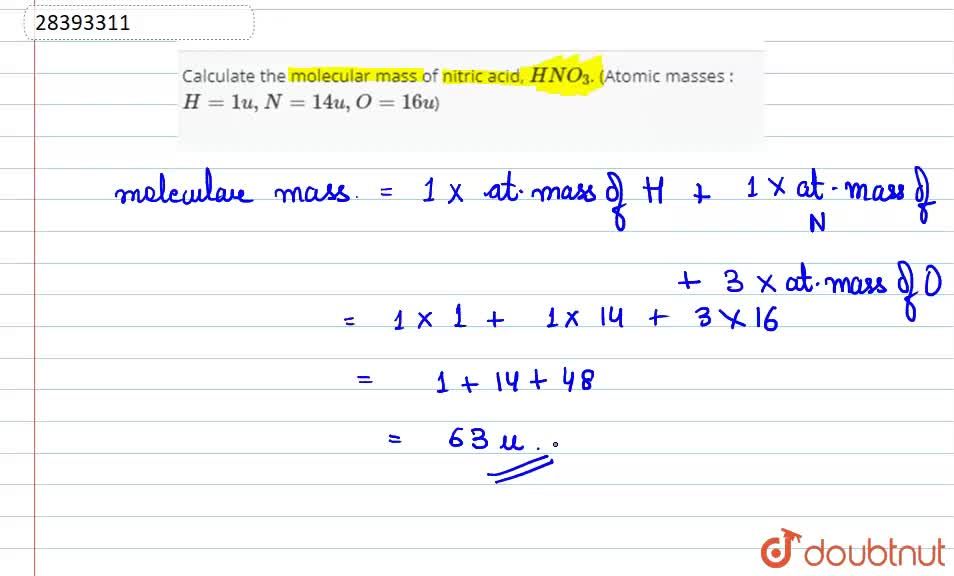
Calculate the molecular mass of nitric acid, `HNO_(3)`. (Atomic masses :` H = 1 u, N = 14 u , - YouTube

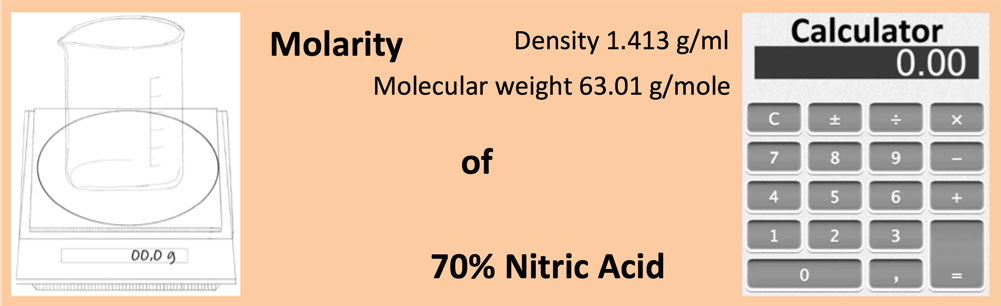
Calculate the concentration of nitric acid in moles per litre in a sample which has a density 1.41 g mL^-1 and the mass per cent of nitric acid in it being 69% .

SOLVED: Question 11 (2 points) A bottle of concentrated nitric acid (HNO3, molar mass 63.01 g/mol; density 1.42 g/mL) is 56.4% HNOz by mass. What is the molality of the aqueous acid

Calculate the concentration of nitric acid in moles per litre in a sample which has density 1.41g/mL - YouTube