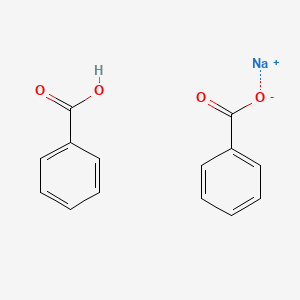
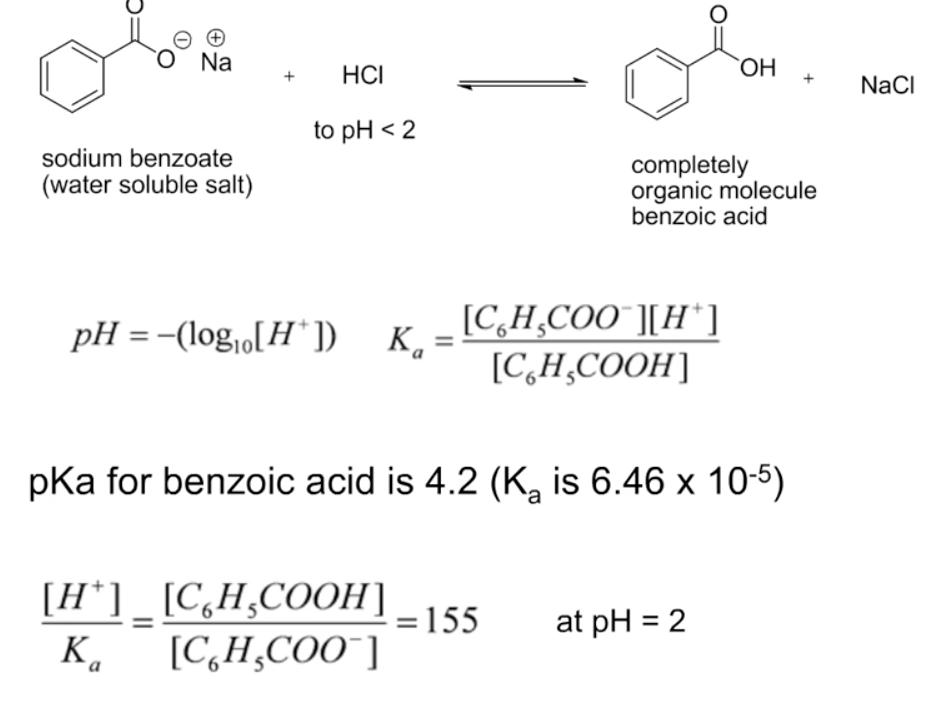
SOLVED: You need to prepare 100.0 mL of a pH 4.00 buffer solution using 0.100 M benzoic acid (pKa 4.20) and 0.200 M sodium benzoate. How many milliliters of each solution should
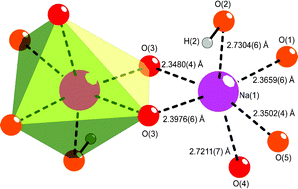
Co-crystallisation of benzoic acid with sodium benzoate: the significance of stoichiometry - CrystEngComm (RSC Publishing)

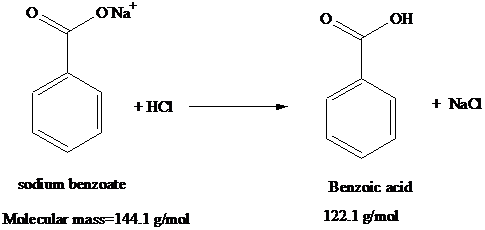
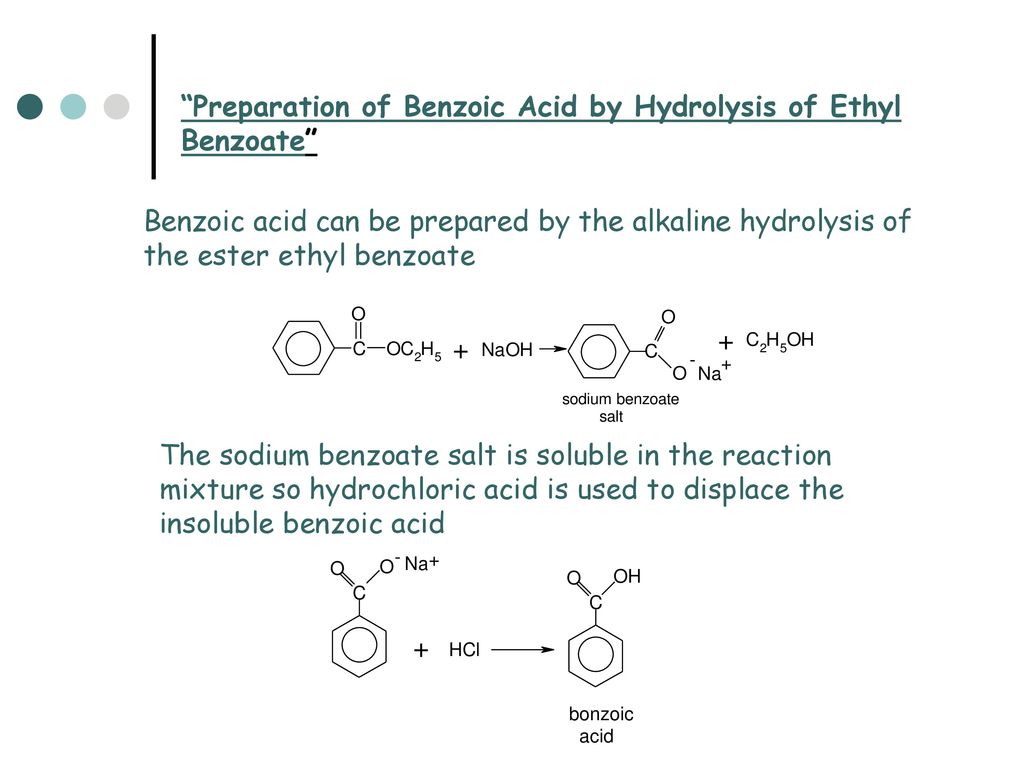
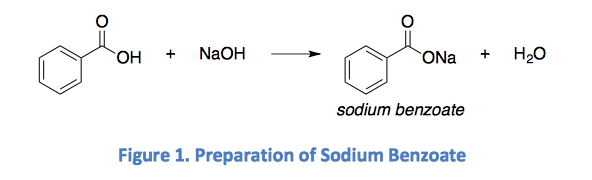
1. Draw a balanced chemical equation for the reaction that would occur between benzoic acid and aqueous sodium hydroxide. 2. Draw a balanced chemical equation for the reaction that would occur between

Create a flowchart for the separation and recovery of benzoic acid and naphthalene. | Homework.Study.com

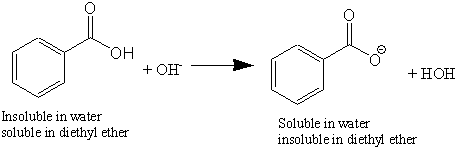
Solved) - Will sodium benzoate be more soluble in water than benzoic acid.... (1 Answer) | Transtutors